The Indecent Act of Ingress: Focusing on Water Chemistry to prevent Cogen Plant failures
This article from longtime industry expert Brad Buecker focuses on the crucial aspects of proper water chemistry to keep those plants running smoothly.
---
For the conventional large coal plants constructed in the last century, and in the heat recovery steam generators (HRSGs) of modern combined cycle power units, the sole purpose of steam production is to drive a turbine or turbines for electricity generation. Turbine exhaust steam condensation in either a water-cooled or air-cooled condenser significantly improves the thermodynamic efficiency of the cycle. The water/steam network approaches closed loop operation unless steam leaks are significant. High-purity makeup water and condensate are a requirement for utility boilers, as otherwise the high temperatures and pressures will induce corrosion or deposit formation. The two primary potential sources of contaminant ingress to utility steam generators are cooling water in-leakage at the condenser (if it is water-cooled), or less common, an upset in the makeup water system. With proper on-line monitoring and personnel trained to recognize and respond to chemistry excursions, major upsets can be minimized. [1]
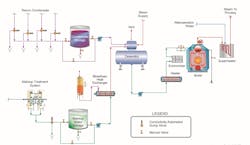
The situation may be significantly different for co-generation plants. Consider the water/steam network outlined in Figure 1 above.
As a preface, it should be noted that many industrial boilers operate at much lower pressures than the typical 2,000 psi or greater of utility units. Thus, higher concentrations of impurities can usually be tolerated in industrial feedwater and boiler circuits. However, increased potential exists for substantial contaminant ingress from condensate return, and sometimes from makeup water systems. For example, consider the case where the author and a colleague investigated a fouling issue at an organic chemicals plant that produced a primary product plus four closely-related derivatives. Steam generation came from several 550 psig package boilers, in which solids deposition in the superheaters, and associated tube overheating, required replacement of the superheater tube bundles every 1.5 to 2 years. Inspection of an extracted superheater bundle revealed internal deposits up to ¼” or so in depth. Furthermore, during our inspection we discovered foam issuing from every saturated steam sample line. The plant had minimal steam generation analytical chemistry equipment, with tests performed by operators, not chemists. Our review of condensate return data provided by the plant’s water treatment vendor showed total organic carbon (TOC) concentrations as high as 200 ppm. Compare this to the recommended upper limit of 0.5 ppm in the ASME industrial steam generator guidelines. [2] The excessive organic contamination in the boilers generated the foam that carried over to the superheaters.
A wide variety of other impurities may enter condensate depending on the chemistry of process fluids that ingress from heat exchanger leaks. These may include mineral salts, acids, bases, and particulates. Such impurities can cause boiler tube corrosion or scale accumulation. Ingress of gaseous compounds may be problematic as well. For example, carbon dioxide that carries over with boiler steam or is admitted elsewhere in the condensate return can generate acidic conditions that induce carbon steel corrosion.
Figure 2. Carbonic acid (H2CO3) corrosion of a carbon steel condensate return line. This and other corrosion mechanisms can in turn release iron oxide particulates that settle in boiler tubes to reduce heat transfer and initiate under-deposit corrosion. Photo courtesy of ChemTreat, Inc.
Makeup System Issues
Another too-common source of industrial boiler impurity ingress is the makeup system. For many years, sodium softening by ion exchange has been a typical choice for makeup water production for lower-pressure industrial boilers. But this author has directly observed, and has seen numerous reports from colleagues, of hardness excursions due to inadequate softener operation and maintenance. Beyond that issue, cases are common where, when the makeup system failed, the operators were directed to bypass the unit and send raw water directly to the boilers. A typical result of hardness excursions is shown below.
Figure 3. Layered boiler tube scale due to multiple hardness incursions. Photo courtesy of ChemTreat, Inc.
Calcium carbonate is the most common scale, but others may be possible including calcium sulfate, and magnesium and calcium silicates. All deposits are strong insulators, and can cause tube overheating and failure.
Figure 4. A bulged and blistered boiler tube from overheating. Photo courtesy of ChemTreat, Inc.
Even with a properly operating softener (and perhaps a downstream decarbonator to remove CO2 from the makeup) many other dissolved ions pass directly to the steam generators. These include chloride, sulfate, and silica. Operating with dissolved solids requires frequent or steady blowdown, which wastes water and energy. Furthermore, upset conditions may arise where the ions generate significant corrosion or scaling. A solution being adopted at some facilities is installation or retrofit of reverse osmosis (RO) as the core makeup treatment method. Even the most basic RO (the single-pass design) will remove over 90 percent of virtually all dissolved ions. Reliable RO design and operation requires detailed raw water makeup analyses and proper selection of pretreatment equipment to protect the RO membranes from fouling.
Condensate, Feedwater and Boiler Water Corrosion Control
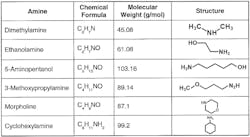
Carbon steel condensate and feedwater piping corrosion is minimized by operation within a mildly basic pH range. Common is feed of ammonia or neutralizing amines for pH conditioning. The latter are small-chain organic molecules with an ammonia group attached to or embedded within the compound.
Table 1. Common neutralizing amines
The equation below illustrates the most fundamental reaction to establish condensate alkalinity, that of ammonia with water.
NH3 + H2O ⇌ NH4 + OH- Eq. 1
Per Reference 2, the recommended feedwater pH range for lower pressure industrial units is 8.8-10.5, which narrows to 8.8-9.6 for higher pressure co-generation systems.
Selecting the best product or product blend is sometimes difficult, as each compound has a different basicity and distribution ratio (the tendency for the product to depart with steam or remain dissolved in the boiler water). It is desirable to have comprehensive pH control throughout the network, but a single compound may not be sufficient to achieve such results. Blended amine products are available that can provide wide-ranging coverage. A thorough analysis of system design, metallurgy, current chemistry, operating temperatures and other parameters is a prerequisite for proper program selection. For example, ammonia in the presence of dissolved oxygen can be severely corrosive to copper alloy heat exchanger tubes.
The boiler offers the harshest conditions within a steam-generating system, and chemistry excursions can easily lead to rapid corrosion or other issues. In power boilers, which typically have consistent high-purity makeup, the primary issue is pH control to minimize carbon steel corrosion. Depending upon boiler type and conditions, a low- to upper-9 pH range is typically recommended.
Tri-sodium phosphate (Na3PO4) is still commonly utilized for pH control in both industrial and many power boilers.
Na3PO4 + H2O ⇌ Na2HPO4 + NaOH Eq. 2
But it is knowledge beyond this fundamental chemistry that may be lacking at plants without trained water/steam chemistry personnel. A prime example is in boilers where hardness and silica may periodically or regularly enter via contaminated condensate. Without proper control, these constituents will form very tenacious calcium and magnesium silicate scales that are extremely difficult to remove. In large measure, it was this scale-forming chemistry that influenced the development of phosphate treatment. Phosphate reacts directly with calcium to produce calcium hydroxyapatite:
10Ca2+ + 6PO43- + 2OH--> 3Ca3(PO4)2•Ca(OH)2 Eq. 3
Magnesium and silica react with hydroxide alkalinity to form the non-adherent sludge, serpentine:
3Mg2+ + 2SiO3- + 2OH- --> 3MgO•2SiO2•2H2O Eq. 4
Calcium hydroxyapatite and serpentine exist as soft sludges and are much easier to remove than the hard scale that would otherwise form. The compounds typically settle in the mud drum or lower headers from which they are removed by blowdown. Careful analyses and evaluation are necessary to design and operate the chemical feed systems to maintain proper conditions. A variety of synthetic polymers have been developed to assist with control of hardness and iron oxide deposition.
Conclusion
This article very briefly touched upon several important issues of co-generation and industrial steam production. Often, the plant staff focuses on process chemistry and engineering to the neglect of water/steam chemistry until a failure occurs. The subsequent loss of production may result in a crisis. Plants may also have specialty waste heat boilers that magnify corrosion and scale formation, sometimes even with seemingly correct chemistry control. [3, 4] Accordingly, I encourage those readers who have found this article to be helpful to consider attending the EUC2W conference detailed at the end of this piece. It offers a chance to learn from many experts and to network with industry colleagues. Information is available at: https://conferences.illinois.edu/eucw
References/Footnotes
1. B. Buecker, “Monitoring of Water and Steam Chemistry for Steam Generators”; Chemical Engineering, September 2019.
2. Consensus on Operating Practices for the Control of Feedwater and Boiler Water Chemistry in Industrial and Institutional Boilers; © 2021, The American Society of Mechanical Engineers, 2 Park Avenue, New York, NY 10016, USA (www.asme.org)
3. Buecker, B., and K. Kraetsch, “Advanced methods for controlling boiler tube corrosion and fouling – Part 1”; Hydrocarbon Processing, October 2021.
4. Buecker, B., and K. Kraetsch, “Advanced methods for controlling boiler tube corrosion and fouling – Part 2”; Hydrocarbon Processing, November 2021.
A Conference for Co-Generation Water/Steam Generation Personnel
The Electric Utility Chemistry Workshop (EUCW) has been hosted every June by the University of Illinois Urbana-Champaign. Originally developed to provide practical information for Midwestern power plant personnel, the workshop became popular with utility chemists and engineers around the country. But, the utility industry is rapidly changing, in large measure due to issues related to climate change and efforts to decarbonize energy production. One consequence is the continued growth of co-generation and combined heat and power (CHP) units that are integrated within large facilities, including refineries, chemical plants, steel mills, educational and health institutions, and many others. These plants often have multiple boilers, some for co-generation and others that produce steam primarily for process heating. Accordingly, the EUCW is evolving to offer the same high-level practical information for diversified steam generation applications, and for related issues including cooling water, service water, and wastewater treatment. Moving forward, a more accurate acronym for the conference is EUC2W to represent the Electric Utility and Co-Generation Chemistry Workshop.
About the author: Brad Buecker is president of Buecker & Associates, LLC, consulting and technical writing/marketing. Most recently he served as Senior Technical Publicist with ChemTreat, Inc. He has over four decades of experience in or supporting the power industry, nearly half of it in steam generation chemistry, water treatment, air quality control, and results engineering positions with City Water, Light & Power (Springfield, Illinois) and Kansas City Power & Light Company’s (now Evergy) La Cygne, Kansas station. Buecker’s experience also includes eleven years with two engineering firms, Burns & McDonnell and Kiewit; and two years as acting water/wastewater supervisor at a chemical plant. He has a B.S. in chemistry from Iowa State University with additional course work in fluid mechanics, energy and materials balances, and advanced inorganic chemistry. He has authored or co-authored over 250 articles for various technical trade magazines, and has written three books on power plant chemistry and air pollution control. He is a member of the ACS, AIChE, AIST, ASME, NACE (now AMPP), and the Electric Utility Chemistry Workshop planning committee. He may be reached at [email protected].
About the Author
Brad Buecker, Buecker & Associates, LLC
About the author: Brad Buecker is president of Buecker & Associates, LLC, consulting and technical writing/marketing. Most recently he served as Senior Technical Publicist with ChemTreat, Inc. He has over four decades of experience in or supporting the power industry, nearly half of it in steam generation chemistry, water treatment, air quality control, and results engineering positions with City Water, Light & Power (Springfield, Illinois) and Kansas City Power & Light Company’s (now Evergy) La Cygne, Kansas station. Buecker’s experience also includes eleven years with two engineering firms, Burns & McDonnell and Kiewit; and two years as acting water/wastewater supervisor at a chemical plant. He also is a member of the editorial advisory board for Water Technology. He has a B.S. in chemistry from Iowa State University with additional course work in fluid mechanics, energy and materials balances, and advanced inorganic chemistry. He has authored or co-authored over 250 articles for various technical trade magazines, and has written three books on power plant chemistry and air pollution control. He is a member of the ACS, AIChE, AIST, ASME, NACE (now AMPP), and the Electric Utility Chemistry Workshop planning committee. He may be reached at [email protected].